We recently confirmed the identity of two planktonic larvae that were each the subject of blog posts last spring. Both larvae were cryopreserved after photographs were acquired and their DNA was extracted at the Oregon Institute of Marine Biology by a student in Svetlana Maslakova’s Marine Molecular Biology class this fall.
Nephtys sp. metatrochophore
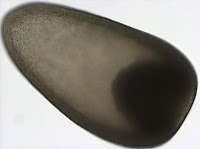
Metatrochophore larvae like this were found in April and May and we speculated that they were larvae of the polychaete genus Nephtys due to the red pigment bands along the prototroch and pygidium as well as the bright blue pigment lining the stomach which you can recall from this photograph (for full blog post see Nephtys sp. metatrochophore by Terra Hiebert).
Jay Bowles, a student in the Marine Molecular Biology class, successfully amplified the 16S rDNA barcoding gene region for this larva with universal primers (Palumbi et al. 1991). Using NCBI BLAST, we compared this sequence to those in GenBank and found that this larva is, in fact, the larva of Nepthys as its sequence matched other species within this genus with 97% sequence similarity. The species level identify of this larva remains to be determined, as sequences did not match exactly, but we’ve confirmed our previous identification based on larval morphology.
Holothuroid doliolaria
I found this larva in a plankton sample taken from the Charleston marina docks on May 17th. We speculated that it may be the larva of a sea cucumber (class Holothuroidea) because of its unique barrel-like shape and transverse ciliated bands (for full blog post see Holothuroid doliolaria by Terra Hiebert).
Jay successfully amplified the Cytochrome Oxidase Subunit I barcoding gene region from this larva, also using universal primers (Folmer et al. 1994). Unlike the Nephtys sequence mentioned above, the sequence we acquired from this larva did not have a close match to any sequences in GenBank. The closest match (87% similarity) was to the sea cucumber Acaudina molpadioides. Richard Emlet (pers. comm.) suspected that our larva may belong to the local holothuroid species, Paracaudina chilensis (rat-tail sea cucumber), based on its morphology and development, so we compared the larval COI sequence to that obtained from P. chilensis by Gustav Paulay (University of Florida, Florida Museum of Natural History). The two sequences had only 4 mismatches over the 490 base pair region of overlap (0.8% divergence). This level of divergence is low enough that we can be confident that our doliolaria larva belongs to P. chilensis.
While pursuing the identification of this larva with DNA sequence data, we realized that we misinterpreted the madreporite as ossicles in the polarized light image you can see here. Calcareous ossicles are found in sea cucumbers and are used for structure and support. However, as we now understand, they usually do not develop until much later stages (Richard Emlet, pers. comm.). The madreporite, on the other hand, is a small calcareous disc that connects the water-vascular system to the outside, and is used to regulate internal water content in all echinoderms, including sea cucumbers. Madreporites have been documented in young Paracaudina sp. larvae (Richard Emlet, unpublished) and resemble the brighter spicule on the right in the photograph shown here. In addition to the madreporite, the water vascular system of echinoderms includes another calcareous structure called the ring canal that helps to move water throughout the body. We speculate that the ring canal can also be seen in this image and is the larger structure to the left of the madreporite.
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3:294-299
Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G. (1991) The simple fools guide to PCR Version 2.0. Honolulu, HI: Department of Zoology Kewalo Marine Laboratory, University of Hawaii