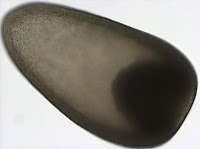
Although this might look like a picture of a flying saucer it is actually a lateral view of a trochophore larva of a polychaete from the genus Polygordius. This larva (and many others like it) were collected from the plankton in Charleston, Oregon on May 10th. The Polygordius trochophore was the first trochophore larva described in the literature (Hatschek 1878), even though it is quite unusual, as far as trochophore larvae go (Rouse 1999). Trochophore larvae, in general, are characterized by having an equatorial ciliary band, called the prototroch. You can clearly see the prototroch in the first picture as a yellow band that divides the larva into two regions - the upper episphere, and the lower hyposphere. The unusual thing about this trochophore, also known as “endolarva” (Woltereck 1904), is that the segmented juvenile body develops tucked inside the spherical larval body. The larva pictured here has a well developed juvenile body inside the transparent hyposphere. The two red spots at the apex of the episphere are the eyes. The greenish/yellow ballon inside the larval sphere is the stomach. Polygordius larvae have a through gut and feed in the plankton.
Although this might look like a picture of a flying saucer it is actually a lateral view of a trochophore larva of a polychaete from the genus Polygordius. This larva (and many others like it) were collected from the plankton in Charleston, Oregon on May 10th. The Polygordius trochophore was the first trochophore larva described in the literature (Hatschek 1878), even though it is quite unusual, as far as trochophore larvae go (Rouse 1999). Trochophore larvae, in general, are characterized by having an equatorial ciliary band, called the prototroch. You can clearly see the prototroch in the first picture as a yellow band that divides the larva into two regions - the upper episphere, and the lower hyposphere. The unusual thing about this trochophore, also known as “endolarva” (Woltereck 1904), is that the segmented juvenile body develops tucked inside the spherical larval body. The larva pictured here has a well developed juvenile body inside the transparent hyposphere. The two red spots at the apex of the episphere are the eyes. The greenish/yellow ballon inside the larval sphere is the stomach. Polygordius larvae have a through gut and feed in the plankton.  This picture offers a view of the larval apical organ located at the apex of the episphere. The two eyes which are part of it appear black in transmitted light. The apical organ also contains two clear vesicles, which look like statocysts. Statocysts are balance organs found in some larval and adult marine invertebrates.
This picture offers a view of the larval apical organ located at the apex of the episphere. The two eyes which are part of it appear black in transmitted light. The apical organ also contains two clear vesicles, which look like statocysts. Statocysts are balance organs found in some larval and adult marine invertebrates.  Polygordius trochophore is one of the few polychaete larvae that have a catastrophic metamorphosis. I was fortunate enough to witness this process in the lab, and took a picture, as the larva was undergoing metamorphosis (left). The orange-yellow band at the anterior end (up) is the prototroch. The process of metamorphosis begins with the juvenile suddenly extending out of the hyposphere. The larval body (including the prototroch) is then resorbed. In this larva the process was more gradual than in some others of this genus - the episphere was resorbed gradually over the course of a few days, and even after a week one could still detect the remnants of the prototroch, even as the now juvenile worm crawled around the bottom of the culture bowl.
Polygordius trochophore is one of the few polychaete larvae that have a catastrophic metamorphosis. I was fortunate enough to witness this process in the lab, and took a picture, as the larva was undergoing metamorphosis (left). The orange-yellow band at the anterior end (up) is the prototroch. The process of metamorphosis begins with the juvenile suddenly extending out of the hyposphere. The larval body (including the prototroch) is then resorbed. In this larva the process was more gradual than in some others of this genus - the episphere was resorbed gradually over the course of a few days, and even after a week one could still detect the remnants of the prototroch, even as the now juvenile worm crawled around the bottom of the culture bowl. Hatschek B. 1878. Studien über Entwicklungsgeschichte der Anneliden. Ein Beitrag zur Morphologie der Bilaterien. Arbeiten aus dem Zoologischen Institute der Universität Wien und der Zoologischen Station in Triest. 1: 277–404.
Rouse G. 1999. Trochophore concepts: ciliary bands and the evolution of larvae in spiralian Metazoa. Biological Journal of the Linnean Society. 66: 411–464.
Woltereck R. 1904. Beiträge zur praktischen Analyse der Polygordius-Entwicklung nach dem “Nordsee”-und dem “Mittelmeertypus”-Typus. I. Die für beide Typen gleichverlaufende Entiwicklungsabschnitt: Vorm E ibis zum jungsten Trochophore-Stadium. Arch. Entw.Mech. Org. 18: 377-403.