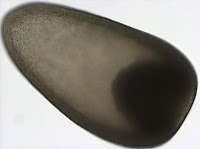
On February 4, 2013, I did a plankton tow off a dock in Charleston, OR
and found a few embryos like the one pictured here. The embryo was about 180
microns across, and was surrounded by an inner chorion and an outer chorion
about 360 microns in diameter. I kept the embryos, and after two days
discovered that they turned into planuliform larvae, hatched from their
chorions and were swimming around in the dish.
 The planuliform larva is
uniformly ciliated and has a prominent bundle of cilia called the apical tuft
at the anterior end (12 o’clock on the second picture). Such larvae are found in
nemerteans (phylum Nemertea, a.k.a. ribbon worms) from the orders Hoplonemertea
and Palaeonemertea.
The planuliform larva is
uniformly ciliated and has a prominent bundle of cilia called the apical tuft
at the anterior end (12 o’clock on the second picture). Such larvae are found in
nemerteans (phylum Nemertea, a.k.a. ribbon worms) from the orders Hoplonemertea
and Palaeonemertea.  After a few more days these
larvae developed features that allowed me to identify them as belonging to the
order Hoplonemertea (e.g. several pairs of subepidermal eyes visible on the
third picture). This picture shows an 11-day old individual. At this point they
mostly crawled on the bottom of the dish, and had developed many of the adult
structures, such as the brain and proboscis, so they can be considered
juveniles rather than larvae. Hoplonemertean metamorphosis (the transition from
planktonic larva to benthic juvenile) is inconspicuous. The transition from
swimming to crawling is accompanied by changes in the epidermis. Apparently
many hoplonemerteans replace the larval epidermis composed of large, ciliated,
cleavage-arrested cells with intercalating smaller cells of the definitive
epidermis (Maslakova and von Döhren, 2009).
After a few more days these
larvae developed features that allowed me to identify them as belonging to the
order Hoplonemertea (e.g. several pairs of subepidermal eyes visible on the
third picture). This picture shows an 11-day old individual. At this point they
mostly crawled on the bottom of the dish, and had developed many of the adult
structures, such as the brain and proboscis, so they can be considered
juveniles rather than larvae. Hoplonemertean metamorphosis (the transition from
planktonic larva to benthic juvenile) is inconspicuous. The transition from
swimming to crawling is accompanied by changes in the epidermis. Apparently
many hoplonemerteans replace the larval epidermis composed of large, ciliated,
cleavage-arrested cells with intercalating smaller cells of the definitive
epidermis (Maslakova and von Döhren, 2009).
To witness this epidermal
transition, I fixed some of my specimens in paraformaldehyde (with a touch of gluteraldehyde)
and stained them with fluorescent phalloidin to visualize the outlines of the
epidermal cells using a confocal microscope.

 The first confocal image
shows a 2-day old hoplonemertean planuliform larva – the same age as the live
larva pictured above. You will notice that large cells dominate the epidermis,
but small cells are visible in between. These small cells are the intercalating
cells of the juvenile epidermis. The bottom image is a 4-day old larva, and you
can see that the large cells of the larval epidermis are farther apart from
each other. The small cells of the juvenile epidermis occupy more space in
between. Eventually, cells of the larval epidermis will be either resorbed or
sloughed off, leaving the cells of the definitive epidermis to cover the entire
surface.
The first confocal image
shows a 2-day old hoplonemertean planuliform larva – the same age as the live
larva pictured above. You will notice that large cells dominate the epidermis,
but small cells are visible in between. These small cells are the intercalating
cells of the juvenile epidermis. The bottom image is a 4-day old larva, and you
can see that the large cells of the larval epidermis are farther apart from
each other. The small cells of the juvenile epidermis occupy more space in
between. Eventually, cells of the larval epidermis will be either resorbed or
sloughed off, leaving the cells of the definitive epidermis to cover the entire
surface.
Maslakova SA, von Döhren J.
(2009) Larval development with transitory epidermis in Paranemertes peregrina and
other hoplonemerteans. Biol Bull 216: 273-292