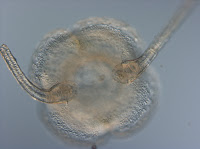
Amphipholis squamata are simultaneous hermaphrodites, which means they produce both eggs and sperm at the same time, and they self-fertilize. The exciting part is that this tiny brittle star is also viviparous. We dissected adults in class to find brooded embryos.
 The long slender arms are made up of a series of flexible joints and can bend and coil. This brittle star will unexpectedly fold up to conceal itself or reach out quickly to make a sneaky escape. In preparation for these photos, I immersed the brittle star in a solution of MgCl2 to anesthetize it. It almost immediately relaxed and stayed still through the subsequent dissection.
The long slender arms are made up of a series of flexible joints and can bend and coil. This brittle star will unexpectedly fold up to conceal itself or reach out quickly to make a sneaky escape. In preparation for these photos, I immersed the brittle star in a solution of MgCl2 to anesthetize it. It almost immediately relaxed and stayed still through the subsequent dissection.
 The long slender arms are made up of a series of flexible joints and can bend and coil. This brittle star will unexpectedly fold up to conceal itself or reach out quickly to make a sneaky escape. In preparation for these photos, I immersed the brittle star in a solution of MgCl2 to anesthetize it. It almost immediately relaxed and stayed still through the subsequent dissection.
The long slender arms are made up of a series of flexible joints and can bend and coil. This brittle star will unexpectedly fold up to conceal itself or reach out quickly to make a sneaky escape. In preparation for these photos, I immersed the brittle star in a solution of MgCl2 to anesthetize it. It almost immediately relaxed and stayed still through the subsequent dissection.  A close view of the aboral side shows the tiny central disc of an adult (4 mm in diameter). The disc isn't strictly circular but pentagonal. The whole body has a five-fold symmetry so there are five (or multiples of five) of every structure, as is characteristic of the phylum Echinodermata, in general.
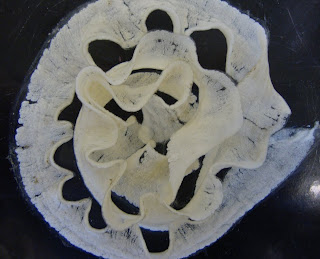
A close view of the aboral side shows the tiny central disc of an adult (4 mm in diameter). The disc isn't strictly circular but pentagonal. The whole body has a five-fold symmetry so there are five (or multiples of five) of every structure, as is characteristic of the phylum Echinodermata, in general. A close view of the oral side shows a smaller pentagon at the center. This is made up of five triangular teeth which, when open, reveal the mouth. On either side of each arm are dark lines. These are bursal slits, entrances to ten sac-like spaces called genital bursae.
A close view of the oral side shows a smaller pentagon at the center. This is made up of five triangular teeth which, when open, reveal the mouth. On either side of each arm are dark lines. These are bursal slits, entrances to ten sac-like spaces called genital bursae.
Ovaries and testes flanking the bursae release sperm and eggs into the bursae through gonopores. Fertilization happens internally and the embryos develop and grow inside the parent's body. The juvenile emerges from the bursal slit when mature. Amphipholis squamata is fertile year-round and is able to brood multiple young at different stages of development.
 Using sharp forceps, I searched for brooded embryos by pulling back the tissue between two bursal slits. After my third try I was pleased to find this tiny juvenile. See also a post by Amanda Clark which shows two dissected juveniles of A. squamata at different stages of development.
Using sharp forceps, I searched for brooded embryos by pulling back the tissue between two bursal slits. After my third try I was pleased to find this tiny juvenile. See also a post by Amanda Clark which shows two dissected juveniles of A. squamata at different stages of development.
 Using sharp forceps, I searched for brooded embryos by pulling back the tissue between two bursal slits. After my third try I was pleased to find this tiny juvenile. See also a post by Amanda Clark which shows two dissected juveniles of A. squamata at different stages of development.
Using sharp forceps, I searched for brooded embryos by pulling back the tissue between two bursal slits. After my third try I was pleased to find this tiny juvenile. See also a post by Amanda Clark which shows two dissected juveniles of A. squamata at different stages of development.